HIV/AIDS | Medical News | Research | Services
Seeking Participants for New SOLAR HIV Treatment Clinical Trial
Published: 03-03-2021 | 2 MIN READ | Author: Prism Health North Texas
Prism Health North Texas (PHNTX), serving the critical role of connecting people living with HIV to care and services, has evolved into a nationally recognized leader delivering high-quality HIV and sexual health services in addition to participating in major clinical research studies. The organization recently announced they are enrolling participants in the SOLAR (Switch Onto Long Acting Regimen) clinical study investigating a switch from daily HIV medications to two injections administered every two months.
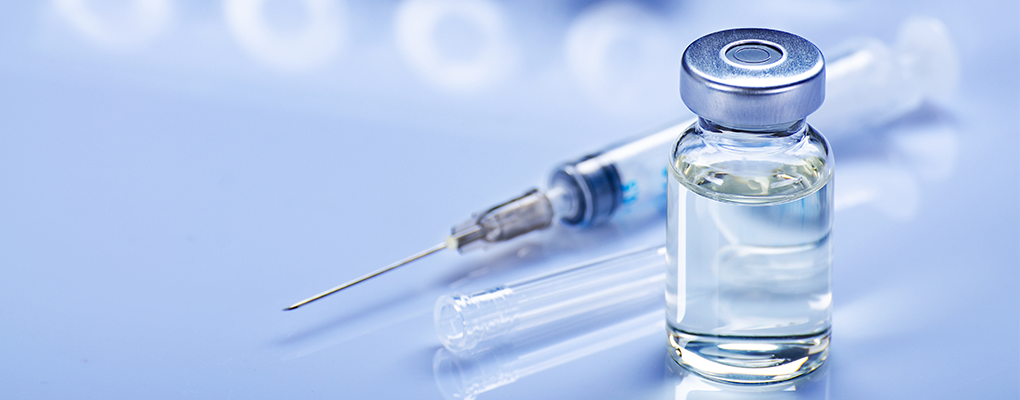
SOLAR is a clinical trial for people living with HIV who are currently doing well on their current single tablet daily oral regimen and might be interested in helping researchers determine if it is safe and effective to switch to a completely injectable, investigational regimen given as two injections every two months. Individuals who have been living with HIV and have been undergoing treatment for at least six months, may qualify to participate in the trial.
“UNAIDS has set the goal of ‘Ending the Epidemic 90-90-90’,” Dr. Gary Sinclair MD, Principal Investigator for SOLAR said. “If 90% of all people living with HIV knew their status, and if 90% of those who know their status were on treatment, and if 90% of those on treatment had complete viral suppression, the HIV epidemic would end.”
Doctors and researchers have long been trying to find HIV treatments that meet the diverse needs of patients. The SOLAR study seeks to determine if this new injectable regimen can maintain the amount of HIV virus at low (undetectable) levels compared to a combination of three HIV medications, taken daily as a single tablet.
Study Details
- Eligible study participants will be assigned at random to one of two options:
- Continue current HIV medications (about 33% of participants), or
- Switch to the investigational medications (about 67% of participants)
- Investigational medications and study-related tests will be provided at no cost
- Study participants will receive compensation for their time, effort, and travel expenses
- Interested participants can learn more by going to phntx.org/research
Provided the injectable regimen is found to be as good or better than the single tablet daily oral regimen, study participants in both groups will be offered the opportunity to continue their HIV therapy with the injectable regimen for up to two additional years, as part of a study extension.
“Current treatments represent amazing advances, but even our best single tablet regimens don’t quite achieve the goals of the 90-90-90 strategy over the long run,” Dr. Sinclair added. “I view studies like SOLAR as a step toward finding treatment options for the remaining patients who struggle to maintain suppression.”
The two investigational medications, long-acting cabotegravir (CAB LA) and long-acting rilpivirine (RPV LA), will be given as a combination. This combination has been approved by the FDA for use in certain patients as a once monthly injectable regimen. SOLAR will continue to investigate this combination in comparison to current HIV treatments. Cabotegravir (CAB) belongs to a class of drugs called integrase inhibitors. Rilpivirine (RPV) is a non-nucleoside reverse transcriptase inhibitor. Both have been studied as oral tablets and long-acting injections.
Learn more and request to be a participant of the SOLAR clinical trial →